Today a small post concerning the synthesis of pyridone starting from pyrone, which is not only a valuable tool in Diels-Alder reaction but also for the preparation of "pyridine" ring.
Iromycins are microbial metabolites which exibit an interesting biological activity as NO synthase inhibitors, their structures are fully substituted pyridones.
Recently, von Zezschwitz et al. from Gottingen in Germany, described the synthesis of Iromycin A and different analogues via cross-coupling reaction.
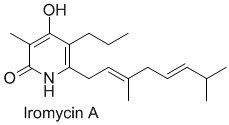
This synthesis of the nucelus starts with the construction of the pyrone ring by successive condensation reactions followed by cyclization. The starting material, the ethyl 2-methyl-3-oxo-butanoate 1 is lithiated by LDA at 0°C, and alkylated with propyl iodide to provide 2 in 56% yield, a second metalation also with LDA give the acetylated intermediates 3 using N-acetylimidazole as acetylating agent. The subsequent lactonization is performed on the crude employing DBU to furnish the desired 2-pyrone 4.

The pyrone in hand, the preparation of pyrone 4 has been realized with aqueous ammoniac in dioxane at 120°C in a sealed tube, through a 1,6-michael addition-ring opening-ring closure procedure in 75%.

Interestingly, the same pyridone can be obtained by a stepwise way, with the same type of reaction in presence of hydrazine, the intermediate hydrazone 5 is hydrolyzed with potassium hydrogenolsulfate to supply the N-aminopyridone 6. The diazotation of 6 in acetic acid lead to the 2-pyrone in 88% (on 2 steps).

Of course, some guys will tell me, this type of reactions are well-known, since "century".
But I like this type chemistry, this is not the usual clockwise metalation of pyridine or synthesis of pyridine by DA reaction.....
Iromycins: A New Family of Pyridone Metabolites from Streptomyces sp. II. Convergent Total Synthesis, Streptomyces sp. II. Convergent Total Synthesis
Heydar Shojaei, Zhen Li-Bo¨hmer, and Paultheo von Zezschwitz, JOC, 2007, 72, 5091.
Iromycins are microbial metabolites which exibit an interesting biological activity as NO synthase inhibitors, their structures are fully substituted pyridones.
Recently, von Zezschwitz et al. from Gottingen in Germany, described the synthesis of Iromycin A and different analogues via cross-coupling reaction.

This synthesis of the nucelus starts with the construction of the pyrone ring by successive condensation reactions followed by cyclization. The starting material, the ethyl 2-methyl-3-oxo-butanoate 1 is lithiated by LDA at 0°C, and alkylated with propyl iodide to provide 2 in 56% yield, a second metalation also with LDA give the acetylated intermediates 3 using N-acetylimidazole as acetylating agent. The subsequent lactonization is performed on the crude employing DBU to furnish the desired 2-pyrone 4.

The pyrone in hand, the preparation of pyrone 4 has been realized with aqueous ammoniac in dioxane at 120°C in a sealed tube, through a 1,6-michael addition-ring opening-ring closure procedure in 75%.

Interestingly, the same pyridone can be obtained by a stepwise way, with the same type of reaction in presence of hydrazine, the intermediate hydrazone 5 is hydrolyzed with potassium hydrogenolsulfate to supply the N-aminopyridone 6. The diazotation of 6 in acetic acid lead to the 2-pyrone in 88% (on 2 steps).

Of course, some guys will tell me, this type of reactions are well-known, since "century".
But I like this type chemistry, this is not the usual clockwise metalation of pyridine or synthesis of pyridine by DA reaction.....
Iromycins: A New Family of Pyridone Metabolites from Streptomyces sp. II. Convergent Total Synthesis, Streptomyces sp. II. Convergent Total Synthesis
Heydar Shojaei, Zhen Li-Bo¨hmer, and Paultheo von Zezschwitz, JOC, 2007, 72, 5091.
2 commentaires:
If you sneeze on a Monday, you sneeze for danger; Sneeze on a Saturday, see your doctor for a
complete physical. 18 scRnd 4: Sc in next sc, dec 1 scover last
2 sc, decrease 1 sc over next 2 sc repeat around.
my site; sexchat
tyudggjh4567
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
golden goose outlet
Enregistrer un commentaire