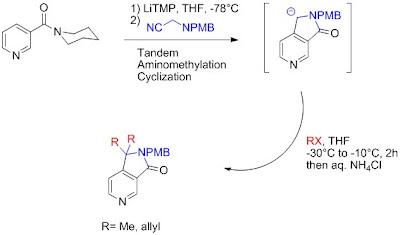
Recently, Bischoff et al. has described the synthesis of 2,3-Dihydropyrrolopyridinone by a tandem aminomethylation-Cyclization using in situ generated formimine and an ortho-lithiated pyridinecarboxamide, depending on the reaction conditions it is also possible to continue the cascade reaction with a bis alkylation of the pyrrolidinone ring.
vendredi 16 février 2007
jeudi 15 février 2007
3-arylquinoline
Side reactions in organic chemistry can be considered as a failure in a target oriented synthesis, but it can be a valuable source a inspiration for the design of new methodologies.
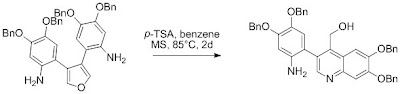
In 2004, Baldwin and coll. tried to synthesize the 5,5',6,6'-tetrahydroxy-3,3'-biindolyl, a potent antioxidant, he proposed as key step a bis-cyclization of the two 2-aminoaryl substituents on the furan (as 1,4-dialdheyde precursor).
 Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Finally, the 3,3'-biindole has been synthesized by a palladium catalyzed homocoupling reaction from the corresponding 3-iodoindole.
For the mechanism of the quinoline formation and other informations see:
Tetrahedron 60 (2004) 3695–3712
In 2004, Baldwin and coll. tried to synthesize the 5,5',6,6'-tetrahydroxy-3,3'-biindolyl, a potent antioxidant, he proposed as key step a bis-cyclization of the two 2-aminoaryl substituents on the furan (as 1,4-dialdheyde precursor).
 Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Finally, the 3,3'-biindole has been synthesized by a palladium catalyzed homocoupling reaction from the corresponding 3-iodoindole.
For the mechanism of the quinoline formation and other informations see:
Tetrahedron 60 (2004) 3695–3712
mardi 13 février 2007
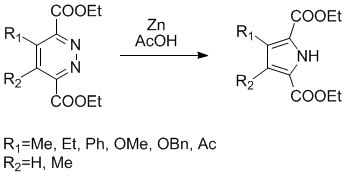
SYNTHESIS OF 3,4-disubstitutedpyrrole-2,5-dicarboxylate
The post of today is an elegant synthesis of tetrasubstituted pyrrole from a pyridazine ring, this synthesis has been described by Dale Boger et al. and require only zinc metal (9-20 equiv. and previously activated(1)) and acetic acid (0.09M), this transformation is performed in about 24h at room temperature with moderate to good yields (42-70%).
 (1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
(1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
1276.
For more information about the procedure and the synthesis of pyridazine see:
Organic Syntheses, Coll. Vol. 9, p.335 (1998); Vol. 70, p.79 (1992).
 (1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
(1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.1276.
For more information about the procedure and the synthesis of pyridazine see:
Organic Syntheses, Coll. Vol. 9, p.335 (1998); Vol. 70, p.79 (1992).
jeudi 8 février 2007
SYNTHESIS OF 3-HydroxyCinchoninic acid
The synthesis of the 3-hydroxycinchoninic acid has been performed with the reaction a commercially available isatin in presence of chloropyruvic acid (previously synthesized from pyruvic acid and sulfuryl chloride in 95-98% Yield.) in a aqueous potassium hydroxyde solution. After the workup the 3-hydroxycinchoninic acid is isolated as a yellow solid(60-71%).

For more information:
Organic Syntheses, Coll. Vol. 5, p.635 (1973); Vol. 40, p.54 (1960).
James Cason and James D. Willett.

For more information:
Organic Syntheses, Coll. Vol. 5, p.635 (1973); Vol. 40, p.54 (1960).
James Cason and James D. Willett.
SYNTHESIS OF 2,3-PYRAZINEDICARBOXYLIC ACID
I will start this blog with a quite old but very interesting synthesis of pyrazine dicarboxylic acid.
This synthesis starts with the formation of quinoxaline, by the reaction of glyoxal and o-phenylendiamine in water with a 85-90% yield(1). Then the benzene fused ring is cleaved with a hot aqueous potassium permanganate solution (2), the mixture is cooled at room temperature and acidified with a 36% hydrochloric acid (3). The crude material is filtered off and recristallized in acetone to give the 2,3-pyrazinedicarboxylic acid with a 75-77% yield.

This synthesis starts with the formation of quinoxaline, by the reaction of glyoxal and o-phenylendiamine in water with a 85-90% yield(1). Then the benzene fused ring is cleaved with a hot aqueous potassium permanganate solution (2), the mixture is cooled at room temperature and acidified with a 36% hydrochloric acid (3). The crude material is filtered off and recristallized in acetone to give the 2,3-pyrazinedicarboxylic acid with a 75-77% yield.

Interestingly, this synthesis can be applied to quinolines, isoquinolines and naphtalenes to provide the corresponding dicarboxylic derivatives.
For more information:
2,3-PYRAZINEDICARBOXYLIC ACID
Organic Syntheses, Coll. Vol. 4, p.824 (1963); Vol. 30, p.86 (1950).
Reuben G. Jones and Keith C. McLaughlin.
Inscription à :
Commentaires (Atom)