Side reactions in organic chemistry can be considered as a failure in a target oriented synthesis, but it can be a valuable source a inspiration for the design of new methodologies.
In 2004, Baldwin and coll. tried to synthesize the 5,5',6,6'-tetrahydroxy-3,3'-biindolyl, a potent antioxidant, he proposed as key step a bis-cyclization of the two 2-aminoaryl substituents on the furan (as 1,4-dialdheyde precursor).
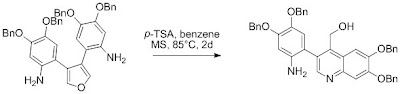
 Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Finally, the 3,3'-biindole has been synthesized by a palladium catalyzed homocoupling reaction from the corresponding 3-iodoindole.
For the mechanism of the quinoline formation and other informations see:
Tetrahedron 60 (2004) 3695–3712
In 2004, Baldwin and coll. tried to synthesize the 5,5',6,6'-tetrahydroxy-3,3'-biindolyl, a potent antioxidant, he proposed as key step a bis-cyclization of the two 2-aminoaryl substituents on the furan (as 1,4-dialdheyde precursor).
 Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Cyclization was attempted with para-toluenesulfonic acid in benzene, and lead to the formation of a 3-arylquinoline in poor 25% yield along with the starting material.
Finally, the 3,3'-biindole has been synthesized by a palladium catalyzed homocoupling reaction from the corresponding 3-iodoindole.
For the mechanism of the quinoline formation and other informations see:
Tetrahedron 60 (2004) 3695–3712
Aucun commentaire:
Enregistrer un commentaire