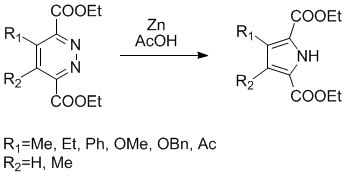
The post of today is an elegant synthesis of tetrasubstituted pyrrole from a pyridazine ring, this synthesis has been described by Dale Boger et al. and require only zinc metal (9-20 equiv. and previously activated(1)) and acetic acid (0.09M), this transformation is performed in about 24h at room temperature with moderate to good yields (42-70%).
 (1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
(1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
1276.
For more information about the procedure and the synthesis of pyridazine see:
Organic Syntheses, Coll. Vol. 9, p.335 (1998); Vol. 70, p.79 (1992).
 (1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.
(1) Fieser, L. F.; Fieser, M. "Reagents for Organic Synthesis"; Wiley: New York, 1967; Vol. 1, p.1276.
For more information about the procedure and the synthesis of pyridazine see:
Organic Syntheses, Coll. Vol. 9, p.335 (1998); Vol. 70, p.79 (1992).
Aucun commentaire:
Enregistrer un commentaire